
| Home | Contact | Technical Glossary | Clients |
 |
|
|
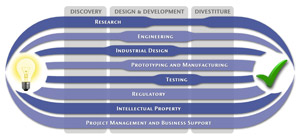
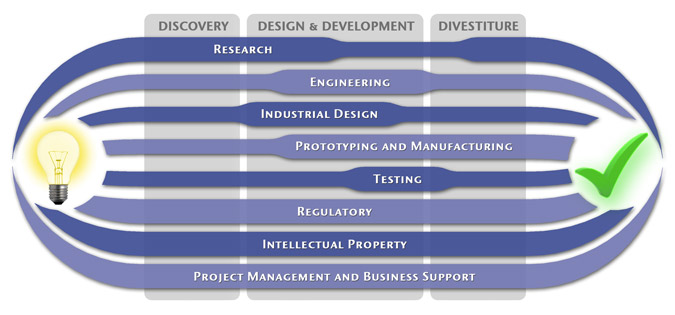
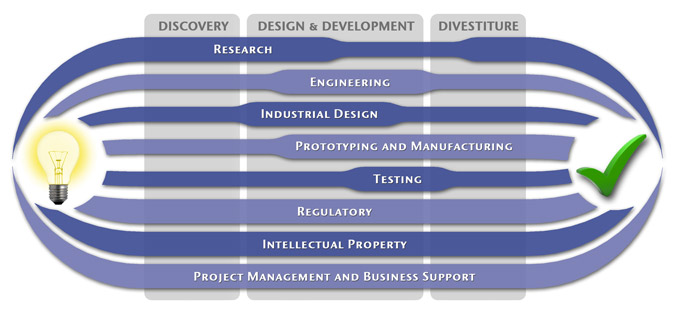
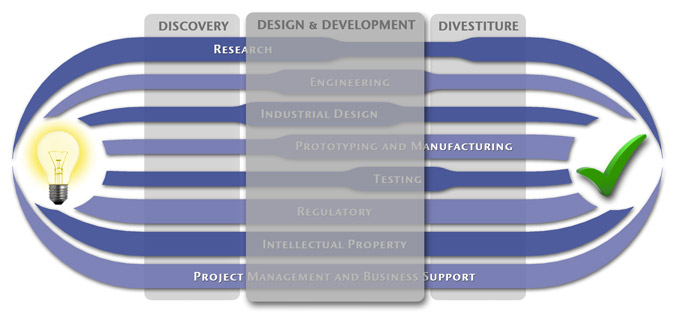
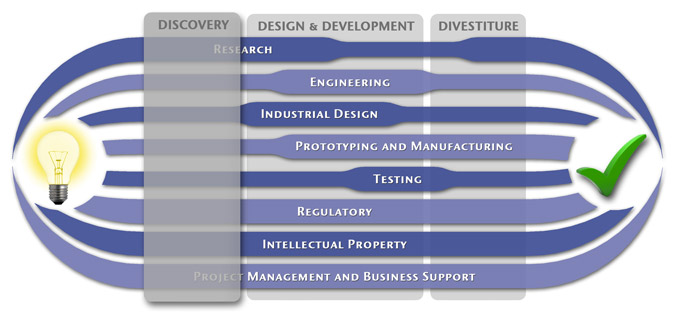
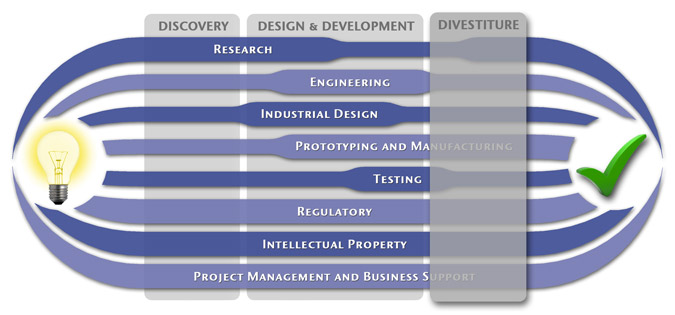
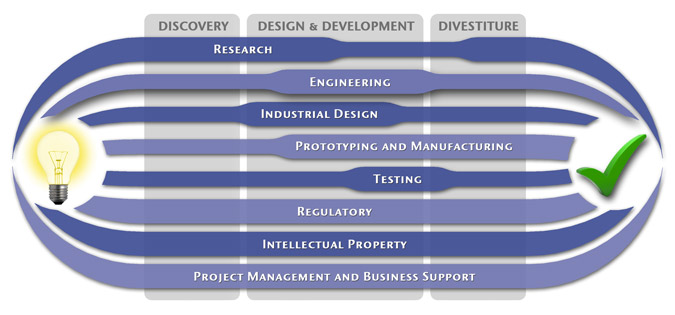
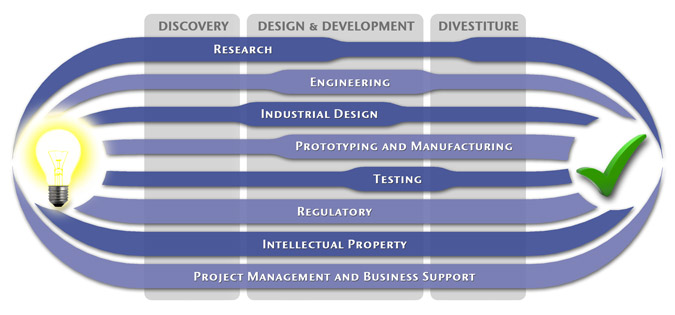
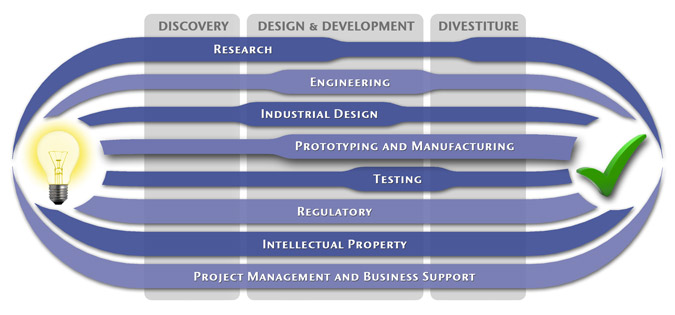
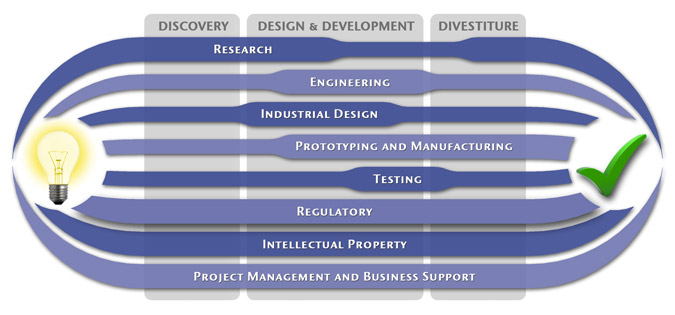
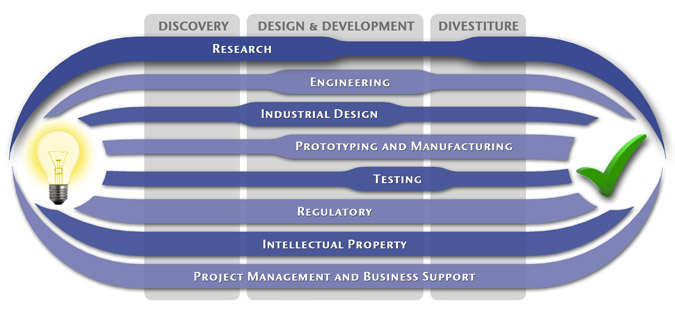
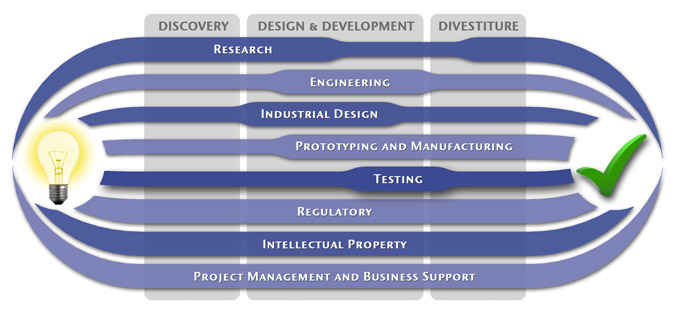
We have a passion for concurrently integrating Industrial Design techniques, Engineering fundamentals, IP Protection, and Manufacturability analysis - yielding products that incorporate aesthetically appealing form, ergonomics, and flawless function. Product Development Engineering
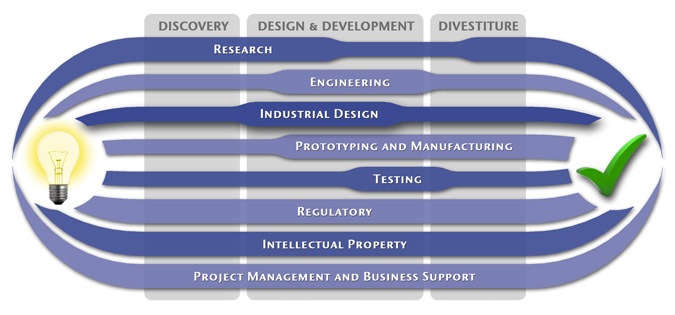
Industrial Design
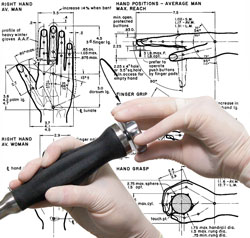
We take functionality to the next level by communicating with you, and focus groups as needed, to optimize the users' modes and methods of interaction with the device. We design products that consider ergonomics to ensure that the user feels as connected and comfortable as possible when using the product. Our Industrial Design Services Include:
Research
To ensure we maximize your product's market share, MedSpark conducts market research, technology assessment, and competition analysis to make sure your design is optimized to capitalize on your competitors' design weaknesses. Prospective, protocol-driven research speeds product development, decreases regulatory approval times, facilitates marketing efforts, and lowers product liability exposure. Verifying your product's Freedom To Operate in the marketplace is one of the most important steps in the Product Development process. At MedSpark we thoroughly analyze the existing patent landscape to minimize the risk of Patent Infringement. Many times it is possible to design around your competitors' patent claims - Something that is only possible if we know they exist. Assessing the needs of your product's users (Voice of the Customer, VoC) is another critical component of successful product development. MedSpark can focus in on your Target Market and determine what their needs are, such as improved mechanical function or enhanced ergonomics. Our Research Services Include:
Prototyping and Manufacturing
Manufacturing Engineering is an integral part of our development process. Its fundamental role in the profitability of a product ensures that it remains an important consideration throughout the product evolution. At MedSpark we have extensive hands-on experience with nearly every commercially available manufacturing technique, which allows for a much more fluid transition between designs on paper and real product. Our Prototyping and Manufacturing Services Include:
Intellectual PropertyYour product is only as valuable as the patents protecting it. We work closely with you to develop and protect your intellectual property rights. Prior Art searches, Freedom-To-Operate Analyses, and Claims language development are performed in concert with experienced patent attorneys to maximize the value of your intellectual property. MedSpark can work with you to license your developed technologies to major manufacturers in exchange for a combination of cash, stock, and/or royalties. Our Intellectual Property Assistance Services Include: 
Regulatory
Wherever you plan to market your new product ideas, we can help you navigate the regulatory hurdles required to achieve your goals. Our Regulatory Assistance Services include:
Testing
Project Management and Business Support
|










































Sending your message. Please wait...
Thanks for sending your message! We'll get back to you shortly.
There was a problem sending your message. Please try again.
Please complete all the fields in the form before sending.