|
Overview
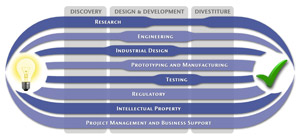
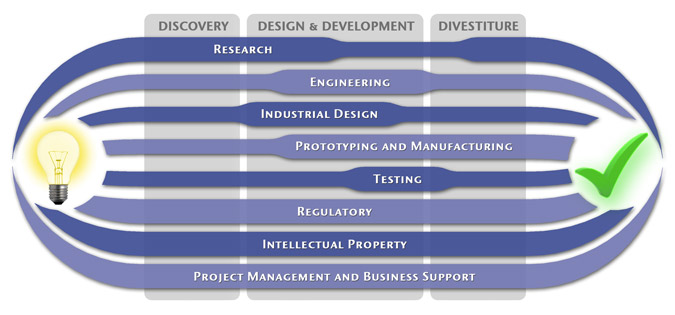
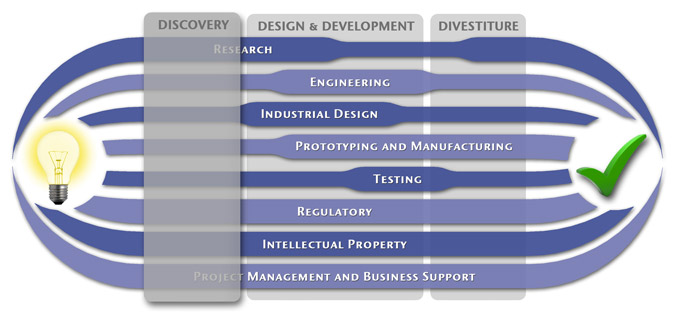
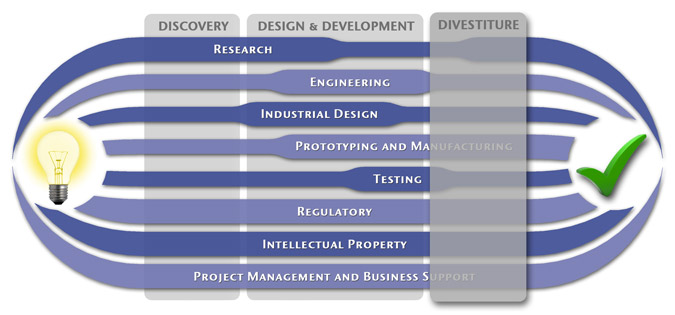
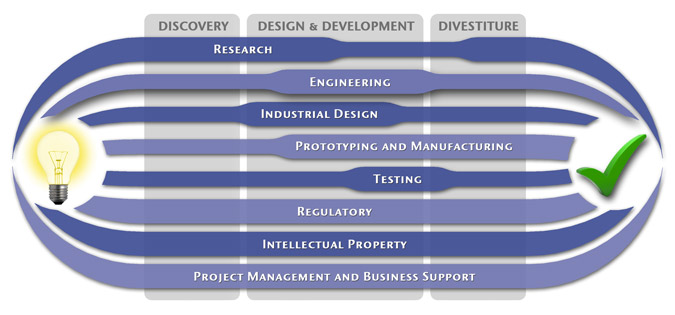
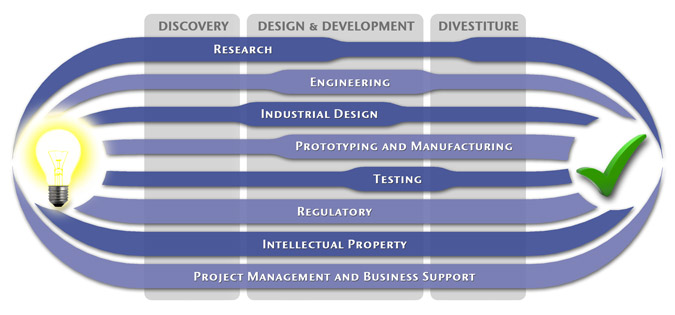
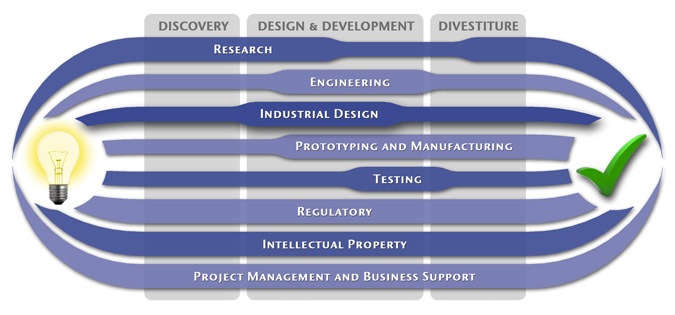
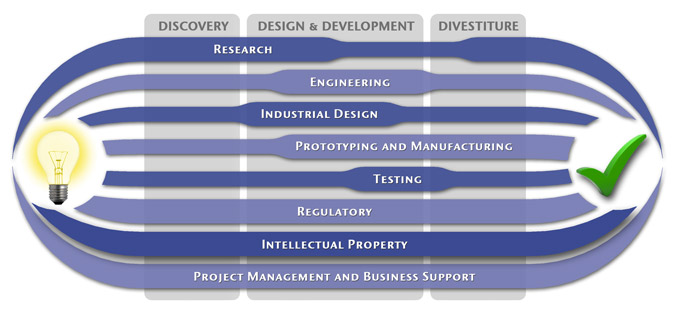
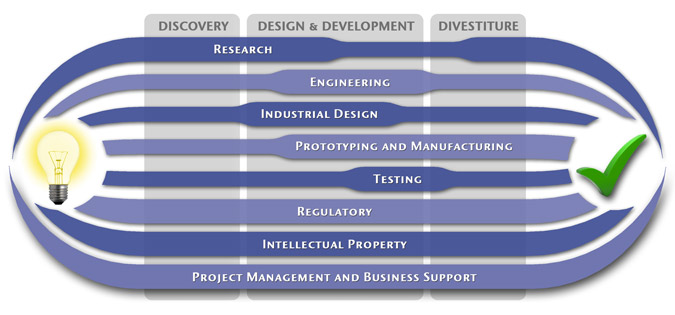
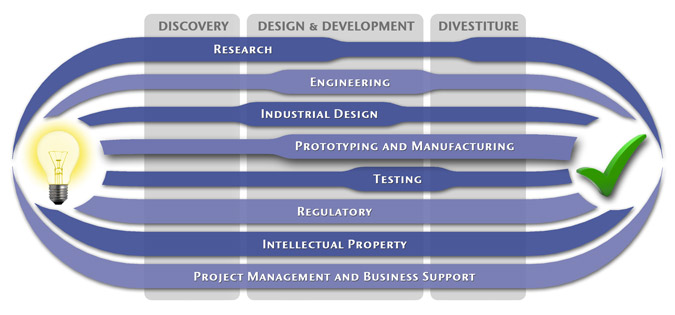
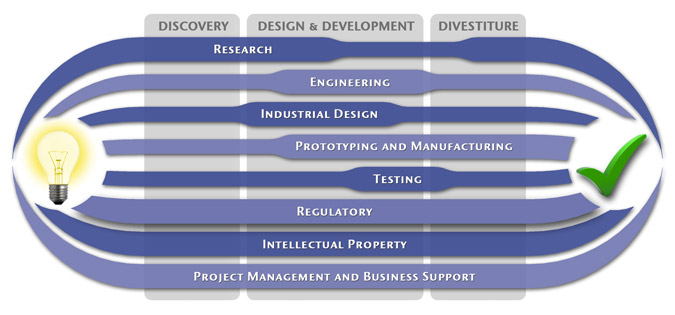
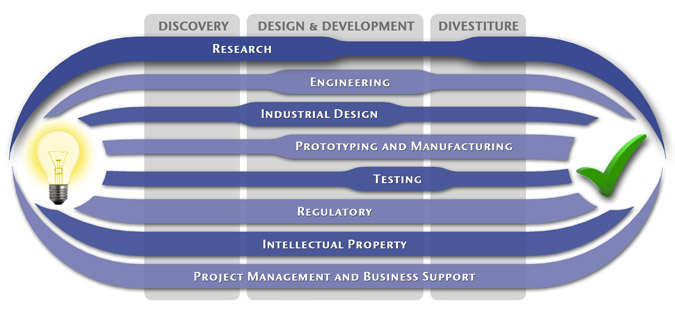
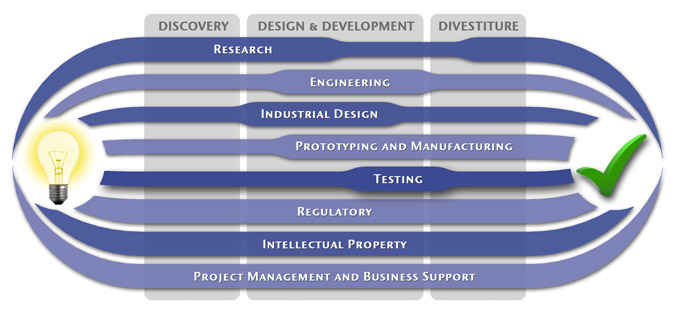
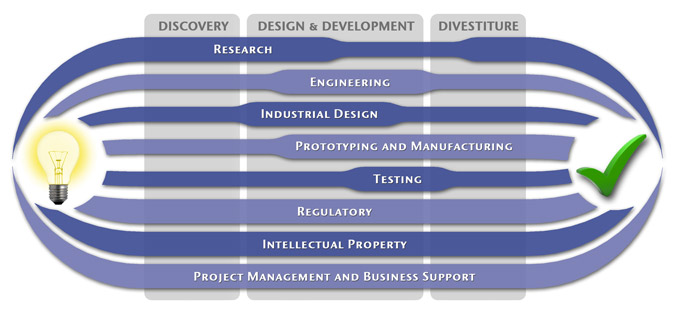
At MedSpark, we approach the product development process as a system of parallel, concurrently applied
foundational services instead of a fixed step-wise sequence. Services such as Engineering, Design, Research,
Intellectual Property, and Manufacturability Analysis are all focused on throughout the entire process based
on the demands of your specific project. In our experience allowing the product development process to be
flexible and dynamic is much more effective than following a strict set of steps. That being said, the
process steps outlined in the graphic above are an example of the development sequence for a hypothetical project.
Your Idea
 Your well-founded idea and hands-on experience in your medical specialty sets the foundation for what could become an exceptional
medical product. Although we’re extremely dedicated to any project we undertake, it’s your commitment and enthusiasm that will
ultimately bring your project goals to fruition.
Your well-founded idea and hands-on experience in your medical specialty sets the foundation for what could become an exceptional
medical product. Although we’re extremely dedicated to any project we undertake, it’s your commitment and enthusiasm that will
ultimately bring your project goals to fruition.
Discovery
 Although possibly the least “exciting” part of the process, the Discovery phase is undoubtedly the most important. In this phase,
several due-diligence activities are performed to ensure there is well-founded purpose in pursuing the commercialization of your
idea. We investigate the degree to which your idea fills a well-established clinical need, will have strong market positioning,
and won't have serious
() concerns.
Although possibly the least “exciting” part of the process, the Discovery phase is undoubtedly the most important. In this phase,
several due-diligence activities are performed to ensure there is well-founded purpose in pursuing the commercialization of your
idea. We investigate the degree to which your idea fills a well-established clinical need, will have strong market positioning,
and won't have serious
() concerns.
If we feel it's an unreasonably risky investment to pursue your idea, we'll be the first to let you know – We only pursue
projects that we feel will ultimately yield a high level of success for our clients. The development process also begins with
formalities such as signing a mutual non-disclosure agreement to protect intellectual property that you've created
and any that will be developed during our process.
- Non-Disclosure Agreement
- Concept Assessment
- Development Questions and Contract Terms
- and Marketability Assessment
- Patentability Assessment
- - Assessment of Current Process, Surgical Procedures, and Needs
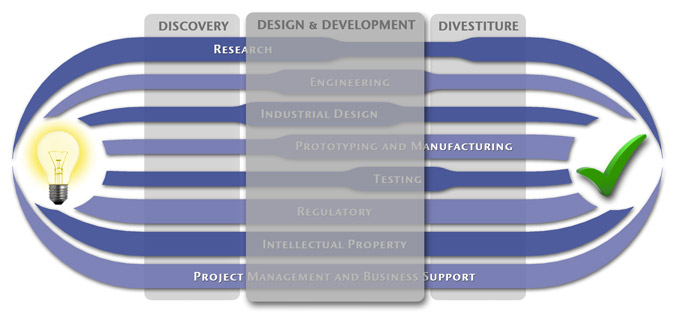
Product Design & Development
 Once we’ve established that your idea has a high likelihood for success, we’ll begin expanding the
concept to consider the possible directions and features that will define the final design. The goal will be for
your finalized design to safely fulfill any clinical needs you intend, be easy to use, reasonably cost effective
to manufacture, and fit well within the existing landscape
– And your product has to do all this while having superior market positioning.
Once we’ve established that your idea has a high likelihood for success, we’ll begin expanding the
concept to consider the possible directions and features that will define the final design. The goal will be for
your finalized design to safely fulfill any clinical needs you intend, be easy to use, reasonably cost effective
to manufacture, and fit well within the existing landscape
– And your product has to do all this while having superior market positioning.
Although the design path, features, form, and manufacturability will continuously be considered starting from
our first meeting, the Product Design and Development phase is where those ideas and concepts are solidified and
realized. Three-dimensional () models are
created to easily visualize the proper function of complex design mechanisms, virtually simulate the inter-relation
of the device and the patient, and evaluate the aesthetic aspects of its form.
With a well-defined design direction and feature set established, we typically write a and file it with the USPTO. Filing a PPA locks-in a date for the current state of design
and acts to prevent competitors from claiming they designed your device first.
Depending on the demands of your particular project, different types of may be built to evaluate
various aspects of the design. Some prototypes are used strictly to see how aesthetical the design will look or
feel in the users’ hands. Other prototypes are utilized to test for adequate fatigue strength, or other
durability/reliability aspects of the design.
- Integrate Discovery Results with Original Concept
- Evaluate Additional Need-Driven Improvement Opportunities
- Engineering - , , , Software, etc.
- - ,
,
- File
- Source and Communicate with Manufacturing Vendors
- Manufacturing - Machining, , Assembly
- Testing - , Environmental, Reliability, , Drop, etc.
Divestiture
 Although it’s an exceptional feeling to help patients and your colleagues by introducing a new product to the
market, the financial rewards that result from your idea and the time and resources you invest in it are an
equally important aspect to your project. There are several ways to divest the technology you’ve created. One
of the primary methods is licensing the technology to another company whereby you receive cash payments and/or
royalties based on mutual agreement. Another divestiture method is to take the product to market yourself.
We’re well-connected in the medical industry and can establish meetings with companies that may be interested
in working with you to either license your technology or provide manufacturing facilities and services to you.
Although it’s an exceptional feeling to help patients and your colleagues by introducing a new product to the
market, the financial rewards that result from your idea and the time and resources you invest in it are an
equally important aspect to your project. There are several ways to divest the technology you’ve created. One
of the primary methods is licensing the technology to another company whereby you receive cash payments and/or
royalties based on mutual agreement. Another divestiture method is to take the product to market yourself.
We’re well-connected in the medical industry and can establish meetings with companies that may be interested
in working with you to either license your technology or provide manufacturing facilities and services to you.
- File Regular Patent Applications - Utility and Design
- Manufacturing Prints
- Develop Marketing Materials
- Locate and Approach Potential Buyers and Investors
- Negotiate Royalty Structures and Licensing Agreements
Fulfillment
 Successfully meeting your product goals means a whole lot more to us than dollar signs and industry notoriety.
The primary reason we're here is to increase the quality of life for patients and the dedicated medical
professionals who care for them. This purpose gives us a strong personal connection to seeing your project through to the end.
Successfully meeting your product goals means a whole lot more to us than dollar signs and industry notoriety.
The primary reason we're here is to increase the quality of life for patients and the dedicated medical
professionals who care for them. This purpose gives us a strong personal connection to seeing your project through to the end.
Product Development Engineering
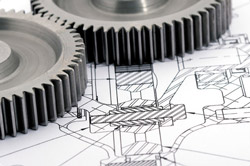
 The MedSpark team can tackle any engineering hurdle from smoothly operating planetary gear systems to electronic systems for handheld
wireless devices. To learn more about our Engineering capabilities, please visit our Services page.
The MedSpark team can tackle any engineering hurdle from smoothly operating planetary gear systems to electronic systems for handheld
wireless devices. To learn more about our Engineering capabilities, please visit our Services page.
Research
 The MedSpark team performs several types of research throughout your product's development cycle to make sure your product is on-track
for success. To learn more about our Research capabilities, please visit our Services page.
The MedSpark team performs several types of research throughout your product's development cycle to make sure your product is on-track
for success. To learn more about our Research capabilities, please visit our Services page.
Industrial Design
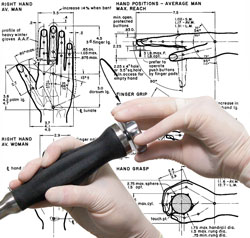 We utilize techniques and methodologies to develop products with improved aesthetics, optimized usability, marketability,
and . To learn more about our Industrial Design capabilities, please visit our Services page.
We utilize techniques and methodologies to develop products with improved aesthetics, optimized usability, marketability,
and . To learn more about our Industrial Design capabilities, please visit our Services page.
Prototyping and Manufacturing
 We will build to validate certain aspects of your design, such as
, mechanical function, user satisfaction, durability,
and reliability. To learn more about our Prototyping and Manufacturing capabilities, please visit our Services page.
We will build to validate certain aspects of your design, such as
, mechanical function, user satisfaction, durability,
and reliability. To learn more about our Prototyping and Manufacturing capabilities, please visit our Services page.
Intellectual Property
 The MedSpark team takes very seriously. searches,
analyses, and writing are performed
to maximize the value of your intellectual property. To learn more about our intellectual property capabilities, please visit our Services page.
The MedSpark team takes very seriously. searches,
analyses, and writing are performed
to maximize the value of your intellectual property. To learn more about our intellectual property capabilities, please visit our Services page.
Regulatory
 We can help you navigate the regulatory hurdles required to introduce your product to the market. To learn more about our Regulatory capabilities,
please visit our Services page.
We can help you navigate the regulatory hurdles required to introduce your product to the market. To learn more about our Regulatory capabilities,
please visit our Services page.
Testing
 Whatever stage of the design cycle, the MedSpark team can manage the testing you need to achieve your product design goals.
To learn more about our Testing capabilities, please visit our Services page.
Whatever stage of the design cycle, the MedSpark team can manage the testing you need to achieve your product design goals.
To learn more about our Testing capabilities, please visit our Services page.
Project Management
 Beyond managing the development of your product's design, we can also assist you with the business-related aspects of your product's success.
To learn more about our Project Management and Business Support capabilities, please visit our Services page.
Beyond managing the development of your product's design, we can also assist you with the business-related aspects of your product's success.
To learn more about our Project Management and Business Support capabilities, please visit our Services page.
|