|
 At MedSpark we're passionate about making people's lives better through innovation. We believe that medical professionals - inspired by their passion to help people - have some of the most insightful new product ideas.
That inspiration, coupled with MedSpark's innovative development process, ignites a creative force that has the power to shape the world of medicine.
At MedSpark we're passionate about making people's lives better through innovation. We believe that medical professionals - inspired by their passion to help people - have some of the most insightful new product ideas.
That inspiration, coupled with MedSpark's innovative development process, ignites a creative force that has the power to shape the world of medicine.
If you have an idea for a product that will create improved outcomes for your patients and colleagues, give us a call and turn that idea into an innovative solution.
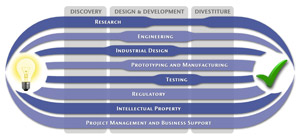
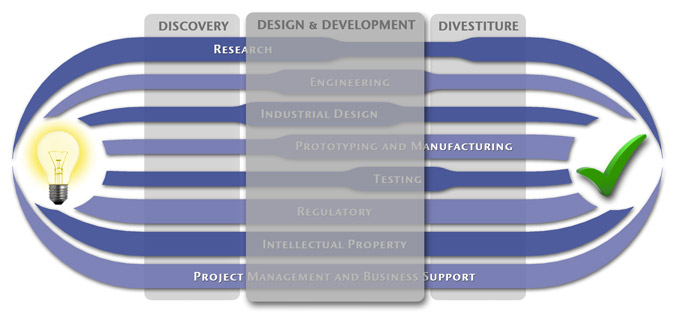
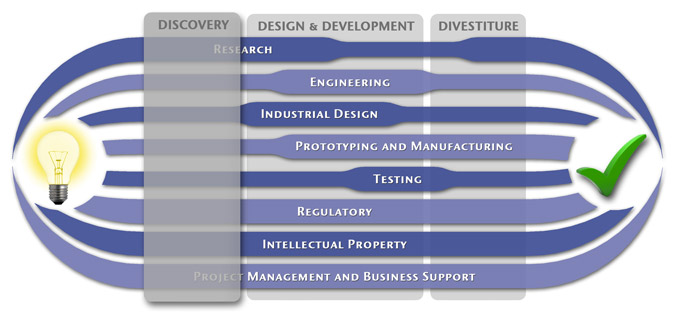
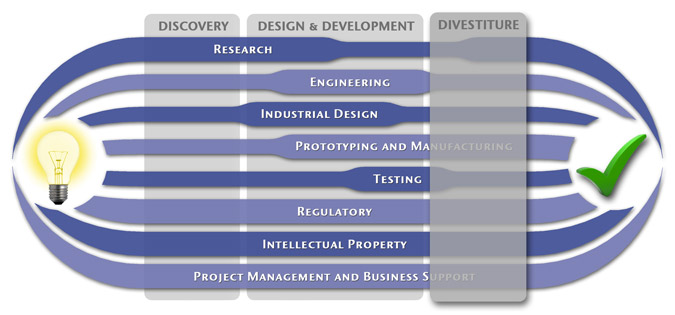
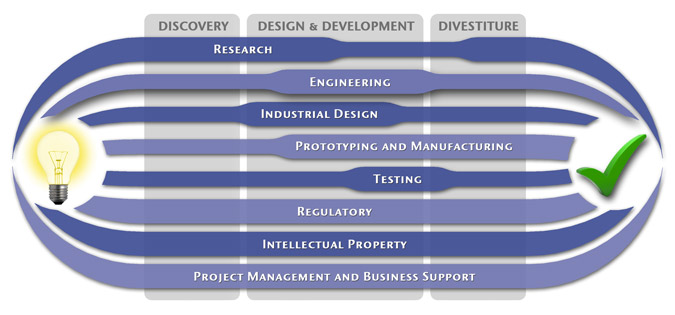
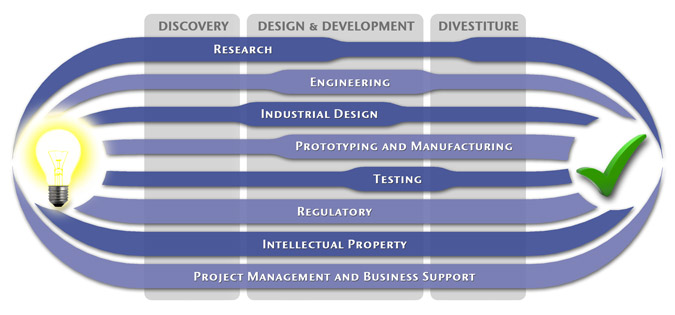
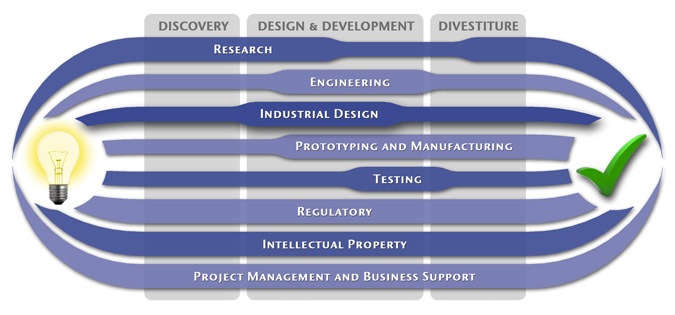
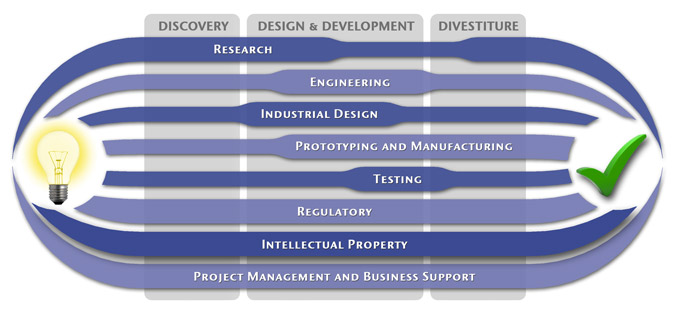
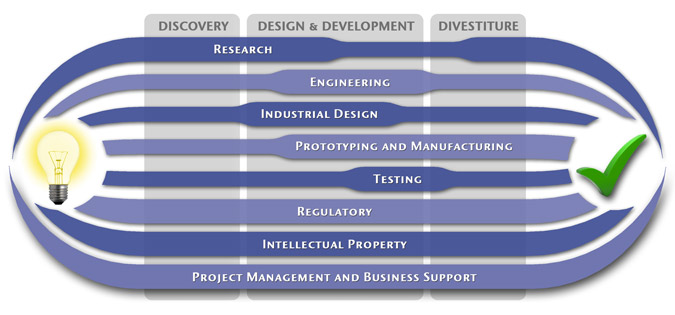
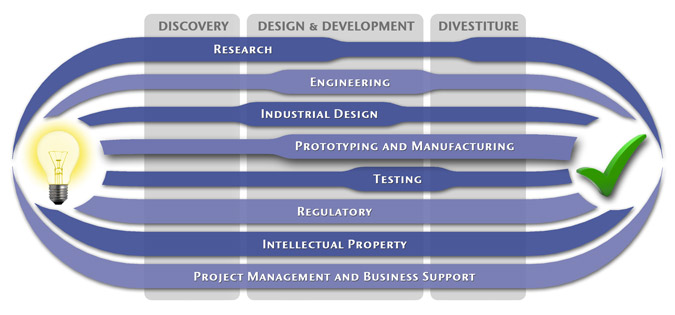
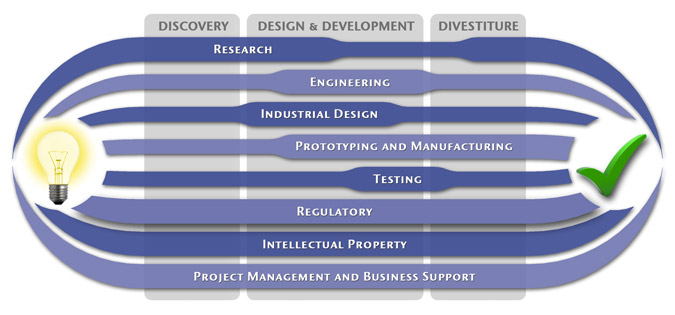
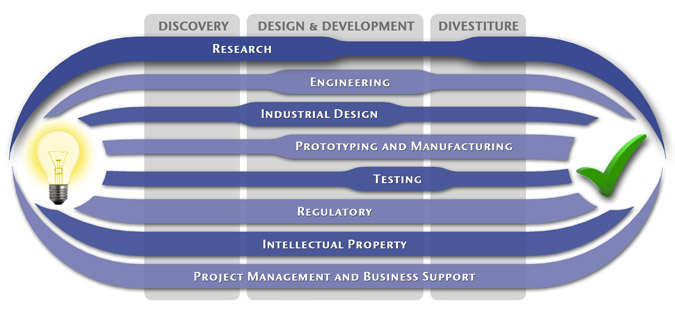
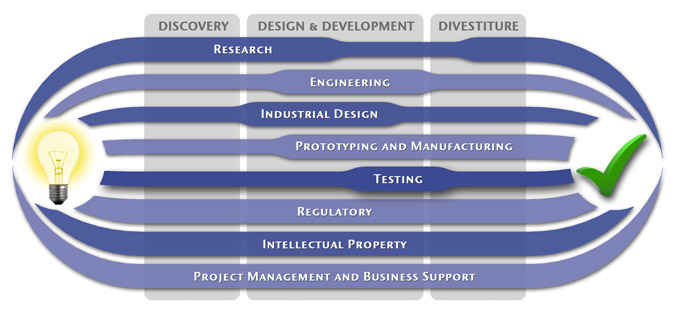
Medical Device Design & Product Development
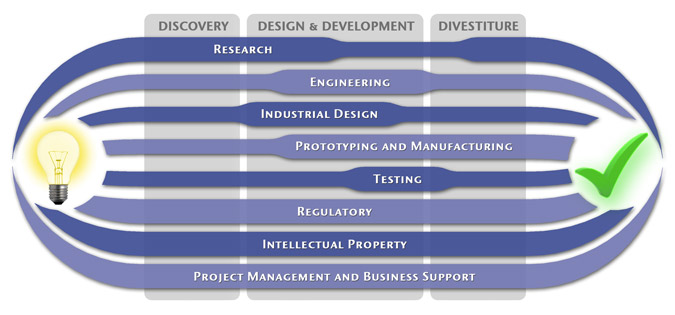
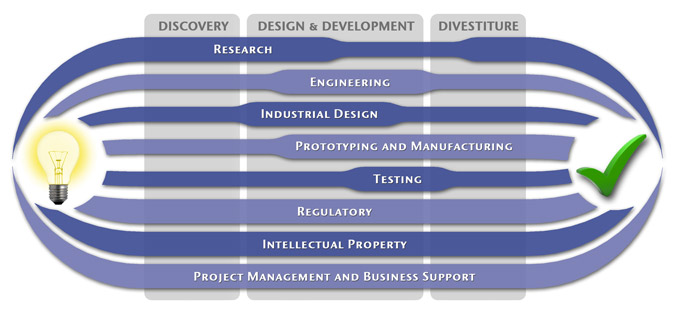
 MedSpark is a medical device design and medical product development firm focused on new product development and innovation. We help medical professionals evolve their medical device inventions and invention ideas into innovative, safe, and effective medical products that meet FDA/CE regulatory requirements. MedSpark is conveniently located in San Luis Obispo, directly between Northern and Southern California, and we serve clients throughout California (e.g. San Francisco, Los Angeles, Orange County, San Jose, Fresno, Sacramento, Long Beach, Oakland, and San Diego CA) and worldwide.
MedSpark is a medical device design and medical product development firm focused on new product development and innovation. We help medical professionals evolve their medical device inventions and invention ideas into innovative, safe, and effective medical products that meet FDA/CE regulatory requirements. MedSpark is conveniently located in San Luis Obispo, directly between Northern and Southern California, and we serve clients throughout California (e.g. San Francisco, Los Angeles, Orange County, San Jose, Fresno, Sacramento, Long Beach, Oakland, and San Diego CA) and worldwide.
- Class I, II, III Medical Device Development: Medical device engineering and design of: Class 1 medical devices, surgical instruments, and durable medical equipment; Class 2 medical devices, surgical implants, biomedical devices, medical instruments, medical equipment; and Class 3 medical implant design.
- Plastic Device Design: Reusable/Autoclavable (steam sterilizable) plastic instrument engineering, plastic disposable device design, and packaging. We utilize manufacturing methods such as: plastic injection molding, liquid silicone injection molding (LIM), rotational molding, thermoforming, compression molding, and reaction injection molding (RIM).
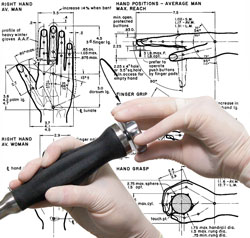
- Industrial Design: Industrial Designers are utilized during the medical product design process to create products that are ergonomic, user centered, aesthetically pleasing, more marketable, and anthropometrically correct.
- Surgical Device Developers: Surgery device and electro-mechanical instrument design
- Prototypes: Medical device prototype manufacturing services include: rapid prototyping (Polyjet, SLA, SLS, FDM, DMLS, EBM, and 3D Printing), machining, welding, prototype development, computer simulation videos, 3D animation, CAD modeling, photorealistic rendering, demonstration model fabrication
- Patents: Patent writing and filing of medical device patent, utility patents, design patents, patent searches (prior art searches), freedom to operate analysis, and patent prosecution.
We regularly coordinate teams of specialized industry professionals, including medical professionals (surgeons, physician assistants, nurses, technicians, therapists, etc),
mechanical engineers, industrial designers, biomedical engineers, lead users, electrical engineers, patent attorneys, electronics engineers, manufacturing engineers, medical
design engineers, regulatory experts, UI designers, plastic device designers, test technicians, medical device engineers, machinists, prototype manufacturers, and sales representatives.
We also partner with engineering firms, contractors, medical device companies, medical equipment companies, graphical user interface (GUI) designers, medical device manufacturers,
medical device manufacturing companies, medical equipment manufacturers, development companies, RAC regulatory affairs consultants, and test labs to take your medical device development
project from concept, through prototyping, medical device testing, qualification, pilot runs and production manufacturing. We also offer all types of testing services including
environmental testing, stress testing, impact testing, fatigue testing, reliability testing, tensile testing, hardness testing, and proof load testing. Talk with a Product Development Engineer today to discuss your development needs.
Areas of Focus
MedSpark is extremely effective and efficient at operating in a wide range of fields within the medical industry.
- Orthopedic Implants, Instruments and Devices
- Arthroplasty (Joint Reconstruction)
- Trauma / Emergency Medicine
- Intensive Care Equipment
- Surgical Implants
- Surgical Instruments
- Arthroscopy (Arthroscopic Surgery)
- Craniofacial
- Pediatrics
- Capital Equipment
- Disposable Medical Products
- ER / Emergency Room Equipment
- Durable Medical Equipment (DME)
- Physical Therapy Equipment / Therapeutic Devices
- Otolaryngological / Otolaryngology / ENT
- Podiatric / Podiatry (Foot and Ankle)
- Hospital Medical Equipment
- Medical Equipment Carts
- Sports Medicine
- Urology
- Anesthesiology
- Biotechnology
- Biomaterials
- Diagnostics and Equipment
|
- Dermatological / Dermatology
- Spine
- Obstetrics / Gynaecology (Ob/Gyn)
- Surgical Equipment
- Biomedical Devices
- Oncological / Oncology
- Radiological / Radiology
- Ophthalmological / Ophthalmology
- Dental
- Orthodontic
- Plastic Surgery
- Cardiology
- Oral and Maxillofacial
|
Class 1, 2 & 3 Medical Devices
- Class I Medical Device Engineering, Design and Development
- Class II Medical Device Engineering/Developers (510k Notification)
- Class III Medical Device Development (As needed - PMA, IDE)
 Specialties: Surgical implants, surgical instrumentation, disposable surgical devices, medical cart design, user interface design (UI design), plastic disposable device engineering, medical
equipment invention, product design and development, handheld (hand held) device design and industrial engineering, diagnostic equipment design, graphical user interfaces, ophthalmic, therapeutic, urological,
orthopedic, intravascular, surgical instrument engineering, medical instrument design, medical equipment engineering , cardiopulmonary, medical instrument engineering, cardiovascular, medical instrument
development, circulatory, surgical instrument design, neurological medical device development, medical device patent, engineering and design, medical equipment development, point of care (POC) device
development, medical equipment design, blood filtration, processing, separation filtering and diagnostic devices, medical device invention, fluid handling, medical product engineering, fluid analysis,
what is product engineering, how to design a product, medical device product development, surgical design, and design development. Specialties: Surgical implants, surgical instrumentation, disposable surgical devices, medical cart design, user interface design (UI design), plastic disposable device engineering, medical
equipment invention, product design and development, handheld (hand held) device design and industrial engineering, diagnostic equipment design, graphical user interfaces, ophthalmic, therapeutic, urological,
orthopedic, intravascular, surgical instrument engineering, medical instrument design, medical equipment engineering , cardiopulmonary, medical instrument engineering, cardiovascular, medical instrument
development, circulatory, surgical instrument design, neurological medical device development, medical device patent, engineering and design, medical equipment development, point of care (POC) device
development, medical equipment design, blood filtration, processing, separation filtering and diagnostic devices, medical device invention, fluid handling, medical product engineering, fluid analysis,
what is product engineering, how to design a product, medical device product development, surgical design, and design development.
Medical Device Manufacturing, and FDA Compliance

- Medical Device Manufacturing:MedSpark can assist you with any scale of manufacturing from prototype medical device manufacturing services to initial pilot production runs, small-scale contract manufacturing, through full production.
- Clinical Evaluation and Testing:We can assist with all aspects of pre-clinical and clinical testing and evaluation including in-vitro testing, in-vivo testing, clinical strategy, clinical protocols, IRB submissions, and creation of design history files (DHF).
- Medical Device Quality, Certification, and Regulatory Compliance:We can perform or coordinate laboratory testing, establishing design controls, certifications, design transfer, compliance, and regulatory affairs. Including, FDA requirements 510k notification, PMA), CSA/UL registration, GMP/cGMPs (Good Manufacturing Practices), Quality Management Systems (QMS), QSRs, CE Mark, ISO Certification (9000, 9001, 9002, 13485), EMC, EMI, IEC 60601 Compliance, and IEC 60601.
Central California Based, Worldwide Services
 Based in San Luis Obispo, CA (directly between Northern and Southern California), we are strategically located to serve customers world-wide. In situations where small segments of a project are outsourced to third parties (such as specialty manufacturing vendors), we’ve forged strategic relationships with companies with the required infrastructure (testing labs, FDA consultants) within Southern/Northern California (CA) region, primarily in the San Francisco Bay Area, Los Angeles (LA), Orange County, Sacramento, San Jose, Fresno, Long Beach, San Diego, and Oakland CA.
Based in San Luis Obispo, CA (directly between Northern and Southern California), we are strategically located to serve customers world-wide. In situations where small segments of a project are outsourced to third parties (such as specialty manufacturing vendors), we’ve forged strategic relationships with companies with the required infrastructure (testing labs, FDA consultants) within Southern/Northern California (CA) region, primarily in the San Francisco Bay Area, Los Angeles (LA), Orange County, Sacramento, San Jose, Fresno, Long Beach, San Diego, and Oakland CA.
Within the US, we have assisted many medical professionals throughout the West Coast (CA, OR, WA, AZ), Midwest (ID, UT, OH, IL), East Coast (ME, KY, NY, NJ, CT, PA) and South West/South East (TX, FL, NC, SC). Within our areas of specialty, we assist international clients in Europe (EU nations, e.g. Germany, UK and France), Africa, Mexico, South America (Argentina and Brazil) and Asia (e.g. China, Japan, India, Malaysia, Taiwan and Singapore).
|